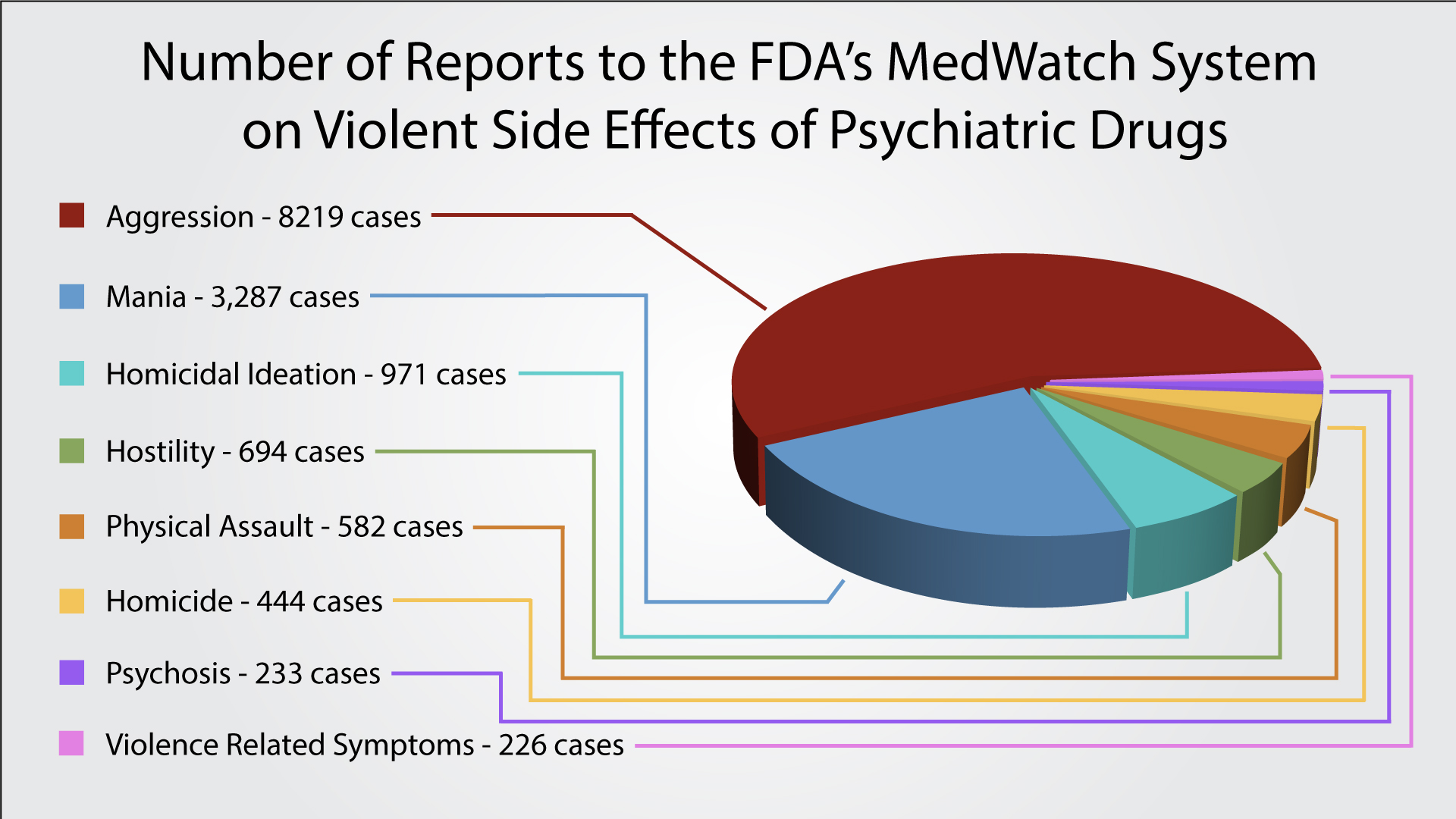
39 warning labels on drugs
325 DOT Hazardous Materials Warning Labels and Markings - USPS The warning labels shown in Exhibit 325.3 a, Exhibit 325.3 b, and Exhibit 325.4 may appear only on mailpieces containing mailable hazardous materials that require use of the label under Postal Service requirements. Division 5.1, 5.2, Class 8 and Class 9 labels are only permitted when used in conjunction with a Limited Quantity air mark. FDA revises labels of SGLT2 inhibitors for diabetes to include warning Mar 15, 2022 · [12-4-2015] A U.S. Food and Drug Administration (FDA) safety review has resulted in adding warnings to the labels of a specific class of type 2 diabetes medicines called sodium-glucose ...
The Importance of Reading Medication Warning Labels Another common warning label on prescriptions is "May cause photosensitivity; avoid sunlight while taking this medication". You may think you are safe because you work in an office and don't spend much time outside, but even the few minutes of direct sunlight you receive while driving can trigger a reaction.

Warning labels on drugs
How To Read And Understand The Warning Labels On Drugs And Medications ... There are four types of warning labels on drugs and medications: 'contraindication', 'precaution', 'interaction', and 'adverse reaction'. These will be discussed further in the next sections. Knowing the types of warning helps you understand the potential risks of using a drug or medication, and how to avoid them. Perceptions of prescription warning labels within an underserved ... Prescription warning labels are small colored stickers placed adjacent to the drug label on a prescription bottle that provides important cautionary information concerning the safe administration of a medicine. For example, "take with food" or "limit time in sunlight when taking this medication". Prescription Labels and Drug Safety - Consumer Reports Warnings typed directly onto patient labels in a large typeface. Research has found that fewer than 10 percent of people examine their drug containers for the colorful warning stickers that...
Warning labels on drugs. Black Box Drugs | FDA Warning Information Dec 08, 2017 · According to a 2006 study, nearly 40% of patients in ambulatory settings were prescribed drugs that had a boxed warning. In some of the recorded cases, pregnant women even received medications where the boxed warning made the drug contraindicated in pregnancy. ... What does this warning mean? Often boxed warning labels have abbreviations … Do Doctors Read Drug Warning Labels? - CBS News The FDA has already banned four drugs that have killed patients because the doctors never read the warnings or disregarded them. Another drug will be removed this summer. Now the FDA is prepared ... pe.usps.com › text › pub52325 DOT Hazardous Materials Warning Labels and Markings ... The warning labels shown in Exhibit 325.3 a, Exhibit 325.3 b, and Exhibit 325.4 may appear only on mailpieces containing mailable hazardous materials that require use of the label under Postal Service requirements. Division 5.1, 5.2, Class 8 and Class 9 labels are only permitted when used in conjunction with a Limited Quantity air mark. USP 800 Labeling Requirements | United Ad Label Shelf labels help communicate that vital information to your staff. In addition, precaution labels applied to the container alert workers to the hazardous drug and its potential harm. Use Appropriate Personal Protective Equipment (PPE) An essential USP <800> safety element is the use of appropriate PPE.
Labeling Information | Drug Products | FDA For prescription drug labeling resources (e.g., Prescribing Information, FDA-approved patient labeling, and carton and container labeling), please see the Prescription Drug Labeling Resources web page A Warning About Warning Labels - U.S. Pharmacist Label Color: Approximately 42% of the patients correlated the label's color to the severity of the message. Patients reported that a red label meant danger, yellow translated to caution, and blue, white, and green labels were viewed as "recommendations" that were not as critical as the instructions on red labels. › news › black-box-drugs-whatBlack Box Drugs | FDA Warning Information Uloric: This gout drug created by Takeda Pharmaceuticals received a black box warning in February 2019, after studies revealed that it contributed to more cardiac-related incidents than its most common competitor, allopurinol. As a result, some people have started filing Uloric lawsuits. Adults Over 50 Often Ignore Prescription Drug Warning Labels The warning labels include instructions such as, "Do not drive while taking this medication," or "Avoid smoking while taking this drug," the Los Angeles Times reports. The study, published in the journal PLoS One, found participants over age 50 were much less likely to pay attention to the warning labels than those ages 20 to 29.
Common and Rare Side Effects for Accutane oral - WebMD More About Drugs and Medications. Pill Identifier; My Medicine; Interaction Checker; Drugs and Medications A-Z; Drugs and Medical Conditions; FDA Labeling for … Academic Journals | American Marketing Association Journal of Marketing (JM) develops and disseminates knowledge about real-world marketing questions useful to scholars, educators, managers, policy makers, consumers, and other societal stakeholders around the world. It is the premier outlet for substantive marketing scholarship. Since its founding in 1936, JM has played a significant role in shaping the content and boundaries of … Who Reads the Drug Warning Labels?- Hormones Matter Perhaps doctors and patients alike are doing the sensible thing in not bothering to read drug warning labels. If the information that they give is arbitrary and they don't help people to assess the actual risk associated with a drug properly, they should be ignored. The only party that the warning labels are truly serving is the drug ... Stronger Warning Labels Needed on Drugs That Make Patients Lose Control ... June 29, 2016. Stronger Warning Labels Needed on Drugs That Make Patients Lose Control. Public Citizen Petitions the FDA for Black Box Warnings on Drugs That Cause Pathological Gambling, Hypersexuality, Compulsive Shopping or Eating, Other Uncontrollable Behaviors
Warning statements for labels and leaflets of certain medicines Details. Warning statements need to be added to the labels and leaflets of certain medicines. The words in this guidance do not need to be used verbatim but that have already been user tested and ...
FDA Black Box Warnings of 8 Very Common Drugs: Read Your Labels Mar 23, 2017 · A black box warning is a consumer warnings with a black border placed on labels calling out the product’s serious health risks—like the one that appears on tobacco packaging. It is the most severe type of warning by the Food and Drug Administration (FDA). ... Here are just 8 of the common drugs in various categories: ...
Medical Billing & Collection Labels - Final Notice, Past Due, Amount Due, Final Notice, Overdue ...
Aleve oral: Uses, Side Effects, Interactions, Pictures ... - WebMD Check all prescription and nonprescription medicine labels carefully since many medications contain pain relievers/fever reducers (aspirin, NSAIDs such as celecoxib, ibuprofen, or ketorolac).
Fluency & Over The Counter Drug Warning Labels understand the information on drug labels (Williams et al., 1995). The FDA recognizes the importance of warning labels and has made multiple rules to improve them. The question is, can the warning labels be more effective? Quality healthcare outcomes depend upon patients' adherence to recommendations and warnings on the labels.
Defective Drug Warning Labels and Off-Label Use - LawInfo An adequate warning label for a drug will include information such as dosage, active ingredients, and known harmful side effects. However, it's often the case a drug is prescribed by a doctor for a use that wasn't intended, or hasn't been publically endorsed by the drug developers. This was the case with Fen-Phen.
Do Drug Labels Overdo Warnings? - WebMD Warnings on Drug Labels In 2006, the FDA set rules to make drug labels easier to read and understand and reduce "overwarning" on drug labels, but this may have had the opposite effect. In the new...
Pharmacy, Medication Warning Labels & Stickers | PDC Never get important medications or directions mixed up ever again with these pre-printed warning labels for medical, pharmacy, and lab applications. ... Communication Label (Paper, Permanent) Drug Discontinued 1-9/16" x 3/8" Yellow - 500 per Roll, 2 Rolls per Box . 1-378. $7.05. Add to Cart.
Flying Under the Influence: Commercial Pilots and Mind-altering Drugs—Playing Russian Roulette ...
› ama-academic-journalsAcademic Journals | American Marketing Association Journal of Interactive Marketing aims to identify issues and frame ideas associated with the rapidly expanding field of interactive marketing, which includes both online and offline topics related to the analysis, targeting, and service of individual customers.
Warning Label Lawsuits | LegalMatch A warning label lawsuit is a lawsuit brought by a consumer of a product. Consumer products include food, drink, drugs, electronic devices, and mechanical devices. Federal law requires product suppliers and manufacturers to provide adequate warning of the dangers the product may pose. Warnings are in the form of a label that describes the dangers.
FDA Label Search You can search for labels by drug name and link to the Library's information resources about marketed drugs. Download All Labels Health information suppliers and others can download all of the...
Prescription Drug Labeling Resources | FDA FDA's Prescription Drug Labeling Resources website provides over 150 labeling resources for the Prescribing Information, FDA-approved patient labeling, and/or carton and container labeling for ...
Boxed warning - Wikipedia In the United States, a boxed warning (sometimes "black box warning", colloquially) is a type of warning that appears on the package insert for certain prescription drugs, so called because the U.S. Food and Drug Administration specifies that it is formatted with a 'box' or border around the text. The FDA can require a pharmaceutical company to place a boxed warning on the …
What Is A FDA Black Box Warning Label - NastLaw Patients and doctors rely on proper warning labels on drugs. Black box warning labels are one way to keep the public safe, but they are often added long after the drug has been on the market and many people have suffered serious side effects. Drugs that caused serious side effects without providing proper warnings are often the subject of ...
› drugs › drug-safety-and-availabilityFDA Drug Safety Communication: FDA strengthens warning that ... The U.S. Food and Drug Administration (FDA) is strengthening an existing label warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) increase the chance of a heart attack or stroke.
438 Drug Warning Label Stock Photos - Dreamstime Close-up of a label on a bottle of prescription medication warning not to consume alcohol while using the drug Customer in pharmacy holding medicine bottle. Woman reading the label text about medical information or side effects in drug store.
Consumer Warning Labels Aren't Working Consumer Warning Labels Aren't Working by Lisa A. Robinson, W. Kip Viscusi, and Richard Zeckhauser November 30, 2016 Warning labels are everywhere. They alert us to the risks of eating unhealthy...
Warning label - Wikipedia A warning label is a label attached to a product, or contained in a product's instruction manual, warning the user about risks associated with its use, and may include restrictions by the manufacturer or seller on certain uses.










Post a Comment for "39 warning labels on drugs"